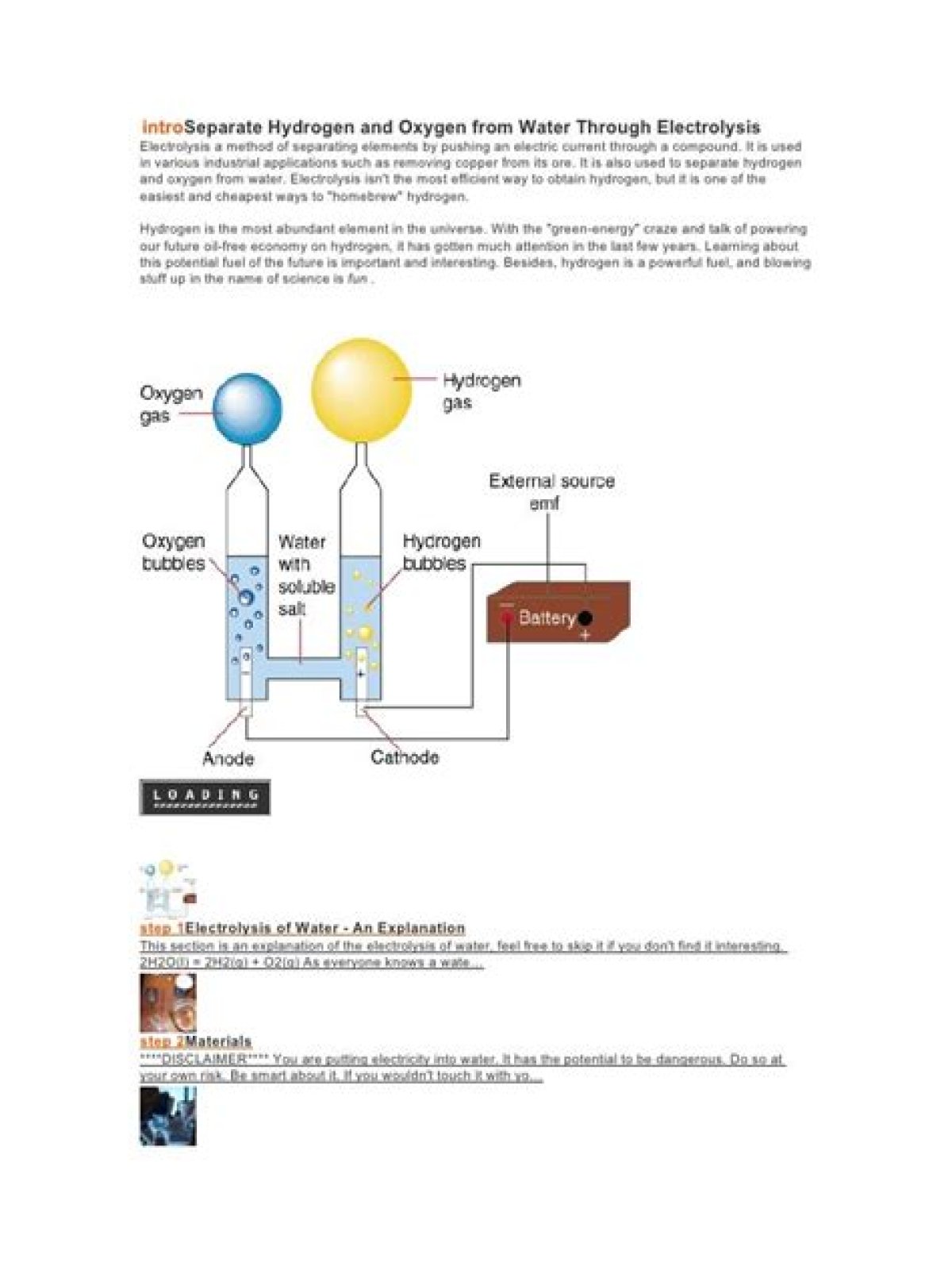
In respect to this, how do you separate hydrogen and oxygen from water?
Splitting the hydrogen and oxygen inwater is accomplished using a process called“water electrolysis" in which both the hydrogenand oxygen molecules separate into individual gassesvia separate “evolution reactions." Each evolutionreaction is induced by an electrode in the presence of acatalyst.
Furthermore, how do you get hydrogen out of water? Electrolysis produces very pure hydrogen fromwater for use in the electronics, pharmaceutical and foodindustries. Relative to steam reforming, electrolysis is veryexpensive. The electrical inputs required to split the waterinto hydrogen and oxygen account for about 80% of the costof hydrogen generation.
Regarding this, how much energy does it take to separate hydrogen and oxygen from h2o?
Re: How much energy is required to split awater molecule into oxygen and hydrogen? Yes,it takes at least as much energy to splitwater into O2 and H2 as is released whenthese gases combine to form water. This is about 260 kJ permole of water or just shy of 5 eV per molecule ofwater (4 electrons times 1.23 V).
Can you extract oxygen from water?
There is such a thing as "artificial gills" thatmechanically extract oxygen from water for a human tobreathe. See also Breathing in oceans full of air. As mentioned inthe first article, it would be possible to separate waterinto oxygen and hydrogen with electrolysis.
What is the splitting of water in photosynthesis called?
How do you separate hydrogen and oxygen from water PDF?
Is decomposing water into hydrogen and oxygen a chemical or physical change?
Can we create water from hydrogen and oxygen?
How dangerous is hydrogen?
How did Stan Meyer's Die?
At what temperature does water separate into hydrogen and oxygen?
How do you separate ethanol and water?
Why does water split in photosynthesis?
How does electrolysis separate hydrogen and oxygen?
How efficient is electrolysis of water?
What is the purpose of splitting water?
How much energy does it take to break the HOH bond within water?
How do you break h2o?
- Power. Electrolysis requires a DC electrical power source.
- Water. Water alone will not conduct electricity.
- Setup. Fill a small tub with your electrolyte solution and thenfill two bottles.
- Electrolysis. Once the experiment is ready, attach the wires tothe battery terminals.